 By
bchristophe
On 19/07/2023
By
bchristophe
On 19/07/2023
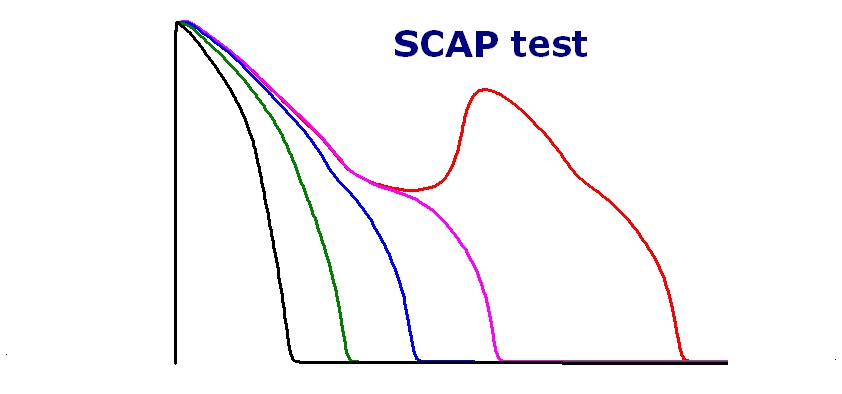
The cardiac safety profiles of 3 new drugs (Ceritinib, Doxepin and Loperamide) are now available (please sign in order to see the result)
The effects of the various compounds were tested on the cardiac action potential of the human cardiac endo-, mid- and epicardial myocyte using the ORd algorithm.
The compound in silico cardiac safety profile was based on search for:
Early afterdepolarization facilitation (EAD)
Transmural dispersion of repolarization increase or decrease (TDR)
Reverse use dependence (RUD)
Triangulation increase or decrease
Action potential duration prolongation or shortening (APD)
Maximal rate of AP rise increase or decrease (Vmax)
qnet increase or decrease (iintegration sum of ICaL+IKr+IKs+INaL+Ito+IK1)
Vmin increase or decrease (minimal rate of AP decrease at the EAD take-off voltage)